Welcome to Kawasaki & Ishii's group at Nagoya Institute of Technology, Japan.
Overview
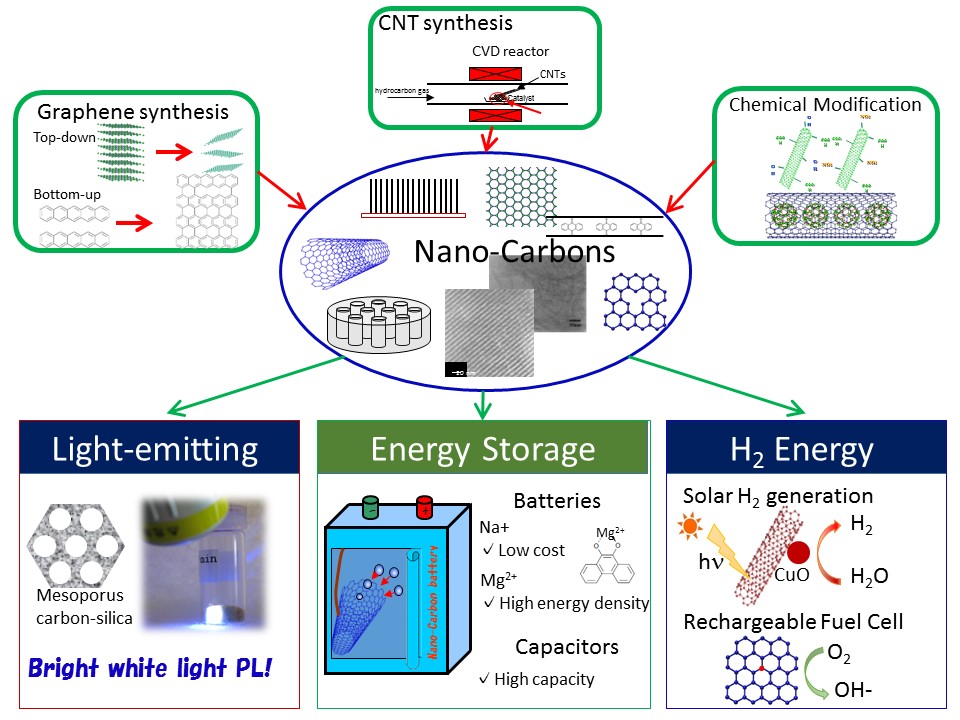
This picture summarizes the recent activities of our lab. In our lab, we are doing synthesis and chemical modification of nanocarbons and nanocarbon composites. The obtained materials are applied to energy relating devices, lighting device, energy storage and energy generation devices.
Synthesis of nanocarbons
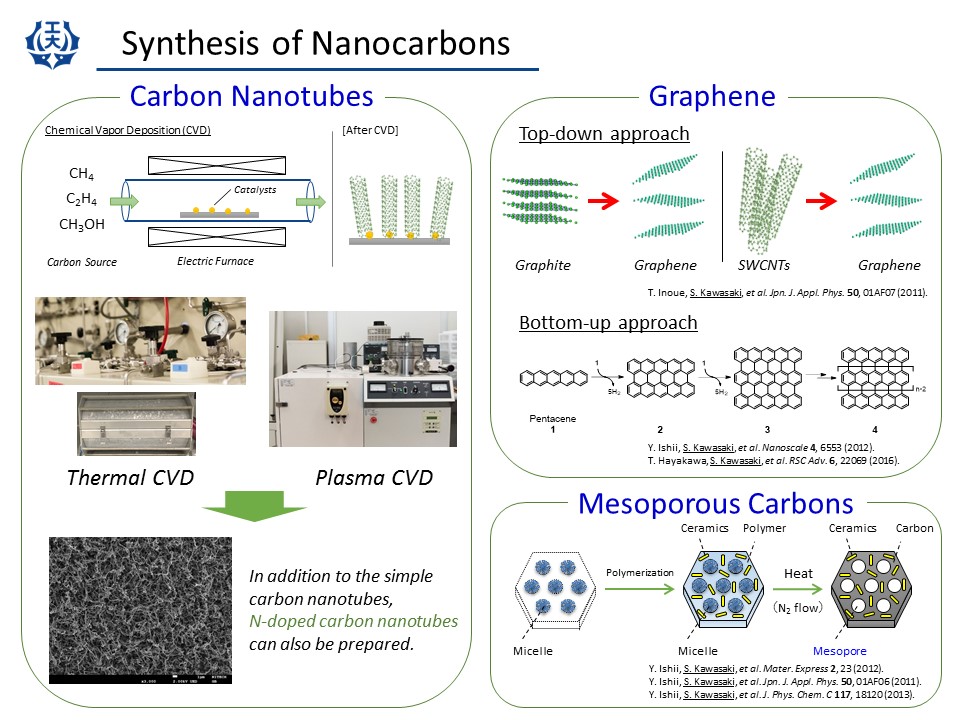
(1) CVD synthesis
We have synthesized single-walled carbon nanotubes (SWCNTs) by thermal CVD. Several kinds of hydrocarbons such as CH4 and alcohols are used as a carbon source. We can also synthesize N-doped SWCNTs by adding nitrogen source such as ethylenediamine.
(2) Graphenes
Graphenes can be prepared by two different approaches: top-down and bottom-up methods. We have prepared reduced graphite oxides by some steps of chemical treatments. We applied similar approach to SWCNTs in order to obtain un-zipped nanotubes. As a bottom-up approach, we prepared graphene nanoribbons by fusing polycyclic aromatic hydrocarbons such as petacene and coronene. (T. Hayakawa et al., RSC adv. (2016), Y. Ishii et al., Nanoscale (2012).)
(3) High Pressure synthesis
High pressure and high temperature treatments of nanocarbons such as fullerenes and nanotubes create new bonds between the molecules/tubes and produce a variety of new phases. We have synthesized some kinds of networked SWCNTs by high-pressure methods.
Modification of SWCNTs
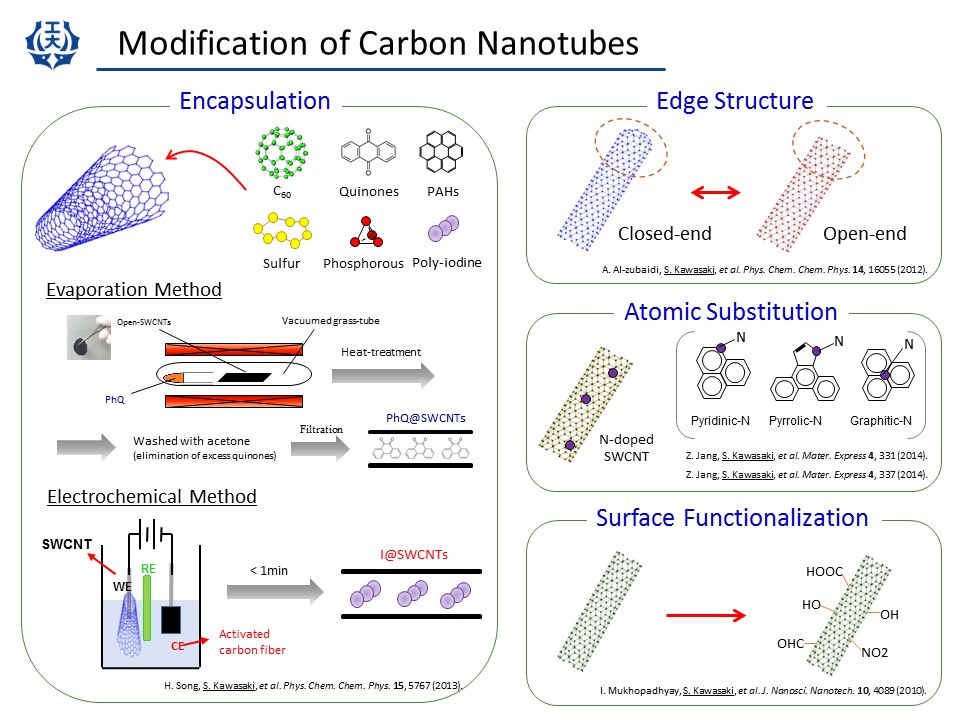
(1) Surface functionalization
The outer surface of SWCNT is chemically very stable because of the long range pi-conjugation system. Then we need to use radical chaemical reaction to introduce some chemical groups on the nanotube surface. We have used physical, chemical and electrochemical methods to introduce several kinds of chemical groups. After the introdcution of the chemical groups we can elongate or substitute the chemical groups. (I. Mukhopadhyay, et al., J. NanoTech. NanoSci. (2010).)
(2) Doping
Nitrogen doping greatly changes the physical and chemical properties of SWCNTs. As mentioned above, we have prepared N-doped SWCNTs by CVD. Not only that, we also have done post-synthesis nitrogen doping to SWCNTs. It was found that post-synthesis doping also improves SWCNT electrode properties for metal-air cell and electric double layer capacitor. (Z. Jiang, et al., Materials Express (2014), Z. Jiang, et al., Materials Express (2014).)
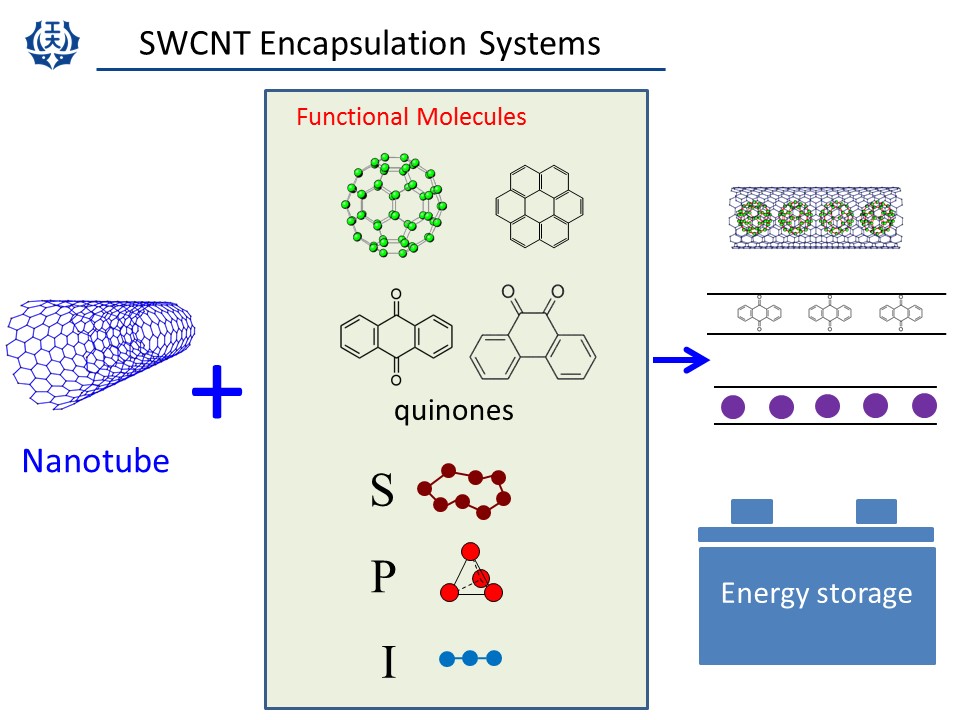
(3) SWCNT encapsulation systems
It was found in 1998 that C60 molecules can be inserted into a hollow core of SWCNT. SWCNTs having C60 molecules are called C60-peapod and have attracted much interests. In our lab., we have inserted several kinds of molecules into a hollow core of SWCNTs.
Energy storage applications
(1) Lithium ion batteries
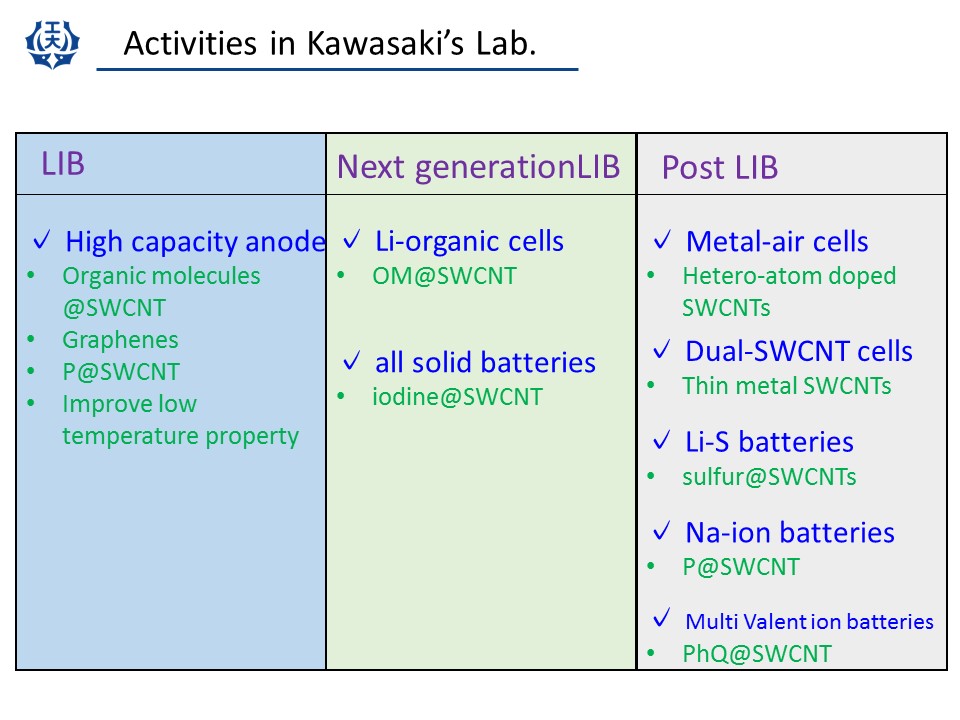
Li ion secondary battery has now become to be an indispesable device for cell phones and laptop computers. The cell energy density of LIB has been improved gradually year by year. The increase of the cell capacity had been achieved mainly by improving the anode (anode materials and packing density). However, the negative electrode capacity is now almost saturated. Therefore, in order to improve the cell capacity, we have to explore new anode materials. For this purpose, we have investigated electrode properties of several kinds of nanocarbons. (S. Kawasaki, et al., Carbon, (2009).)
(2) Post LIBs (Na-ion battery, All solid state battery, Metal-air cell, etc.)
LIB is a very good secondary battery having the highest energy density among the practical batteries. However, of course, it has some problems: safety, cost problems. Furthermore, we have to improve capacity and power performance of LIBs. We expect that SWCNT encapsulation systems shed light on a new way to develop post LIBs. (Y. Ishii, et al., PCCP (2016), Y. Ishii, et al., AIP adv. (2016).)
(3) Electric double layer capacitors (EDLCs)
Since some of nanocarbons have very high specific surface area, nanocarbons have been expected to work as good electrode materials of EDLCs. However, the detailed ion adsorption mechanism of nanocarbons has not been clarified well. In 2012, we reported "dumbbell shape CV" of SWCNTs which reflects unique electronic structure of SWCNTs. (A. Al-zubaidi, et al., JPC C (2012).) We investigate the ion adsorption of SWCNTs by Raman and quartz crystal microbalance measurements. Recently, we reported a paper describing electrolyte redox capacitor using SWCNT encapsulation systems. (Y. Taniguchi, et al., J. Nanosci. Nanotechnol. (2016).)